Jochen Balbach, second project
Prof. Jochen Balbach

Institute for Physics/Biophysics
Betty-Heimann-Straße 7
06120 Halle (Saale)
phone: +49 (0) 345-55 28550
jochen.balbach@physik.uni-halle.de
Tobias Gruber

PhD student
Structural investigations of a membrane curving BAR domain
All members of the protein domain superfamily BAR (Bin, Amphiphysin, RVS167) are important regulators in eukaryotic membrane remodeling processes such as vesicle budding and fissing during clathrin-mediated endocytosis. They are involved in clathrin-coated pits formation and in the biogenesis of T-tuble in muscles. Human Amphiphysin2/Bin1 (hAm2/Bin1) contains a banana-shaped N-BAR domain and an SH3 domain with unknown function connected by a long linker region (see scheme 1). The goal of the project is to study full-length hAm2/Bin1 in structural terms and its membrane remodeling properties by fluorescence based tabulation assays as well as electron and fluorescence microscopy.
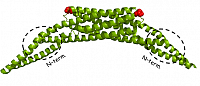
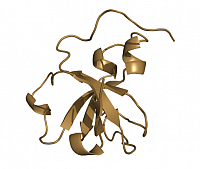
Structure of the N-BAR domain of human Amphiphysin2/Bin1 (top) and the SH3 domain of rat Amphiphysin2/Bin1 (bottom)




